News
Novel coronavirus (COVID-19) and personal protective equipment guidelines March 04 2020
With increasing numbers of cases of COVID-19 virus identified globally and in the United States, the World Health Organization (WHO) and Centers for Disease Control (CDC) are releasing guidelines on screening, personal protective equipment and caring for patients with the novel coronavirus (see below).
In addition to COVID-19, we are still in the midst of cold and flu season. We recommend the following general guidance for all healthcare employees and the general public:
- Wash hands frequently with soap and water for at least 20 seconds or use an alcohol-based hand rub with at least 60% alcohol. Always wash hands when visibly soiled.
- Wash hands prior to touching your eyes, nose, or mouth.
- When in contact with those who are sick, wear the appropriate personal protective equipment
The CDC and WHO are reporting increased demand and plans for surge manufacturing globally to prevent supply shortages.
StethoBarrier is not currently experiencing supply shortages for any of our disposable stethoscope covers. We will update our news section should this change.
Links to resources on the COVID-19 outbreak from credible sources are accessible below:
CDC Healthcare Supply of Personal Protective Equipment
https://www.cdc.gov/coronavirus/2019-ncov/hcp/healthcare-supply-ppe.html
CDC Healthcare personnel guidelines for caring for patients with COVID-19
https://www.cdc.gov/coronavirus/2019-ncov/hcp/caring-for-patients.html
WHO site on the Coronavirus disease (COVID-19) outbreak
https://www.who.int/emergencies/diseases/novel-coronavirus-2019
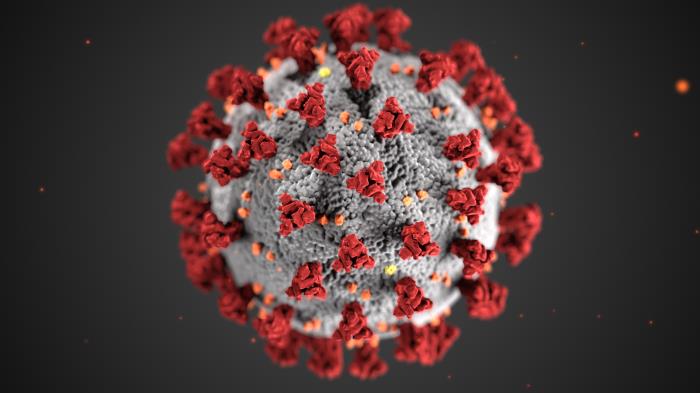
Photo credit:
CDC/ Alissa Eckert, MS; Dan Higgins, MAM. Centers for Disease Control and Prevention's Public Health Image Library (PHIL), identification number #23312.
This illustration, created at the Centers for Disease Control and Prevention (CDC), reveals ultrastructural morphology exhibited by coronaviruses. Note the spikes that adorn the outer surface of the virus, which impart the look of a corona surrounding the virion, when viewed electron microscopically. A novel coronavirus, named Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2), was identified as the cause of an outbreak of respiratory illness first detected in Wuhan, China in 2019. The illness caused by this virus has been named coronavirus disease 2019 (COVID-19).
Centers for Disease Control and Prevention Releases Updated Guidelines for Prevention of Surgical Site Infections May 08 2017
The Centers for Disease Control and Prevention recently released updated guidelines for prevention of surgical site infections. This is a highly anticipated update as the last set of guidelines was released in 1999. As noted in the invited commentary in JAMA, “Surgical Site Infection Prevention—What We Know and What We Do Not Know,” by Dr. Pamela A. Lipsett, “there is a lot of opportunity to learn how we can provide more effective care to our patients.”
While evidence-based medicine remains the gold standard, unfortunately, in many instances there is lack of quality evidence utilizable for evaluating the benefits, harms and tradeoffs of various interventions. While there are definitive recommendations in the guidelines, the authors conclude by stating that the proposed evidence-recommendations should be incorporated into comprehensive quality improvement programs in order to improve patient safety. StethoBarrier strongly commends the Centers for Disease Control and Prevention for their efforts to systematically review the literature and concisely summarize their recommendations.
The findings and conclusion from the report is excerpted below, please use the link in the full citation below to access the full report.
Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017
Findings Before surgery, patients should shower or bathe (full body) with soap (antimicrobial or nonantimicrobial) or an antiseptic agent on at least the night before the operative day. Antimicrobial prophylaxis should be administered only when indicated based on published clinical practice guidelines and timed such that a bactericidal concentration of the agents is established in the serum and tissues when the incision is made. In cesarean section procedures, antimicrobial prophylaxis should be administered before skin incision. Skin preparation in the operating room should be performed using an alcohol-based agent unless contraindicated. For clean and clean-contaminated procedures, additional prophylactic antimicrobial agent doses should not be administered after the surgical incision is closed in the operating room, even in the presence of a drain. Topical antimicrobial agents should not be applied to the surgical incision. During surgery, glycemic control should be implemented using blood glucose target levels less than 200 mg/dL, and normothermia should be maintained in all patients. Increased fraction of inspired oxygen should be administered during surgery and after extubation in the immediate postoperative period for patients with normal pulmonary function undergoing general anesthesia with endotracheal intubation. Transfusion of blood products should not be withheld from surgical patients as a means to prevent SSI.
Conclusions and Relevance This guideline is intended to provide new and updated evidence-based recommendations for the prevention of SSI and should be incorporated into comprehensive surgical quality improvement programs to improve patient safety.
Full citation:
Surgeons of the US Navy demonstrating maintenance of a sterile field.
Impact of healthcare provider awareness on hand hygiene June 18 2016
We have long known that healthcare associated infections lead to negative outcomes, increased costs and dissatisfaction with the healthcare system, yet there are many occasions when providers fail to wash their hands and sanitize small medical equipment. As healthcare providers, we never intend to do harm to our patients or actively think to not take precautions. Rather, we are focused on fulfilling other responsibilities and tasks pertaining to patient care and in a rush or moment of forgetfulness we neglect infection control basics. These lapses highlight the importance of a systems-based approach to ensure infection control practices are routinely implemented.
At StethoBarrier we view our products as a visual reminder of stethoscope hygiene and believe that offering a safe alternative to low quality disposable stethoscopes will lead to fewer protocol violations where providers use their own stethoscope on contact isolation patients.
Henry Ford Health System recently took an interesting approach of showing staff members the millions of bacteria that can grow off of common surfaces including unused gloves, doorknobs, a nurse station mouse, health-care workers' hands, a mobile phone and an ultrasound machine.
Their study was inspired by a previous study that was designed to provoke a feeling of disgust in healthcare workers as a means of motivating them to wash their hands. Following posting of the images, the study found that every participating unit experienced at least an 11% increase in hand-washing, with one unit improving by nearly 50%.
Read more on CNN, “This is what made doctors wash their hands” by Janissa Delzo.

Photo Credit: CNN, Bacteria found on at ultrasound machine
Influenza, Stethoscopes and Infection Control September 28 2015
As the fall and winter months arrive we will begin to see influenza cases around the United States. Remember, your stethoscope is a critical fomite in infection control. See below for CDC recommendations on how to protect patients from Influenza during their physician’s visits:
“Fundamental Elements to Prevent Influenza Transmission
Preventing transmission of influenza virus and other infectious agents within healthcare settings requires a multi-faceted approach. Spread of influenza virus can occur among patients, HCP, and visitors; in addition, HCP may acquire influenza from persons in their household or community. The core prevention strategies include:
- administration of influenza vaccine
- implementation of respiratory hygiene and cough etiquette
- appropriate management of ill HCP
- adherence to infection control precautions for all patient-care activities and aerosol-generating procedures
- implementing environmental and engineering infection control measures.
Successful implementation of many, if not all, of these strategies is dependent on the presence of clear administrative policies and organizational leadership that promote and facilitate adherence to these recommendations among the various people within the healthcare setting, including patients, visitors, and HCP. These administrative measures are included within each recommendation where appropriate. Furthermore, this guidance should be implemented in the context of a comprehensive infection prevention program to prevent transmission of all infectious agents among patients and HCP.”
Prevention Strategies for Seasonal Influenza in Healthcare Settings: Guidelines and Recommendations. CDC. Available at: http://www.cdc.gov/flu/professionals/infectioncontrol/healthcaresettings.htm
Further resources available at:
Siegel J, Rhinehart E, Jackson M, Chiarello L, the Healthcare Infection Control Practices Advisory Committee. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in healthcare settings. 2007. http://www.cdc.gov/ncidod/dhqp/pdf/guidelines/isolation2007pdf.